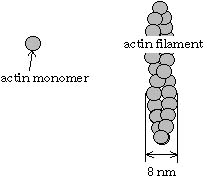
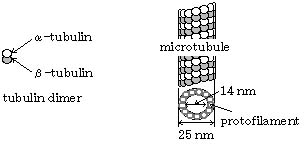
Fig. 1 Schematic structures of actin filament
(left) and microtubule (right)
Actin filament and microtubule, which belong to the cytoskeleton, are found in all eukaryotic cells, and are essential in many cellular functions, e. g. cell division, intracellular transport, cell morphology, cytoplasmic organization, force generation. Actin filament and microtubule consist of actin molecule and tubulin molecule, respectively (Fig. 1). In the cells, several accessory proteins bind to these filament proteins. Our group is studying about structure and function of these accessory proteins.


Fig. 1 Schematic structures of actin filament
(left) and microtubule (right)
(1) Biomolecular motor
(2) Actin-regulated protein
ADF/cofilin family is a low-molecular-mass
actin filament-depolymerizing proteins. In vitro experiments demonstrated
that ADF/cofilin binds to filamentous actin in a 1:1 molar ratio of ADF/cofilin
to actin monomer, and severs and depolymerizes the filaments. In
eukaryotic cells, the ADF/cofilin family is an essential group of actin
regulatory proteins that enhance the turnover of actin by regulating the
rate constants of polymerization and depolymerization.
(3) Microtubule-associated protein
Microtubule-associated proteins
(MAPs) copolymerize with microtubules through in vitro cycles of assembly
and disassembly, stimulate their polymerization, and stabilize the polymer.
MAPs are believed to regulate the diverse and dynamic functions of microtubules
in vivo. MAPs are classified into two groups: neural MAPs and non-neural
MAPs. Three major neural MAPs (MAP1, MAP2, and tau) and a non-neural
MAP (MAP4) have been identified and well characterized at the biochemical
level.
Techniques